Even before the Food Safety and Modernization Act (FSMA) was enacted back on January 4, 2011, the petfood industry was very well aware that sweeping reforms were coming about. We all heard that FSMA was a call for prevention-oriented food safety practices in the US and that FSMA would be the most significant change in food regulation since the establishment of the Food and Drug Administration (FDA) under the Food, Drug and Cosmetics Act in 1938.
The process of proposed rulemaking by FDA would occur through a series of proposed rules published in the Federal Register that would affect the way our companies do business. The most important (to us) would be the proposed rule governing animal feed. We were also very well aware that we, as the petfood industry, would have an opportunity to respond to the proposed rules in an effort to help FDA create the most effective regulations possible to help our industry continue to make safe petfood.
In the months prior to the release of the animal food rule, members of the Pet Food Institute (PFI) began preparing to respond to these new proposed regulations from FDA. In public statements prior to publication of the animal food rule, FDA indicated that the rule would be very similar in most respects to the human food rule, which was published in January 2013. With this knowledge, PFI members began forming teams of experts organized by subject matter in order to study and evaluate specific sections of the human food rule in anticipation of publication of the proposed rule for animal food.
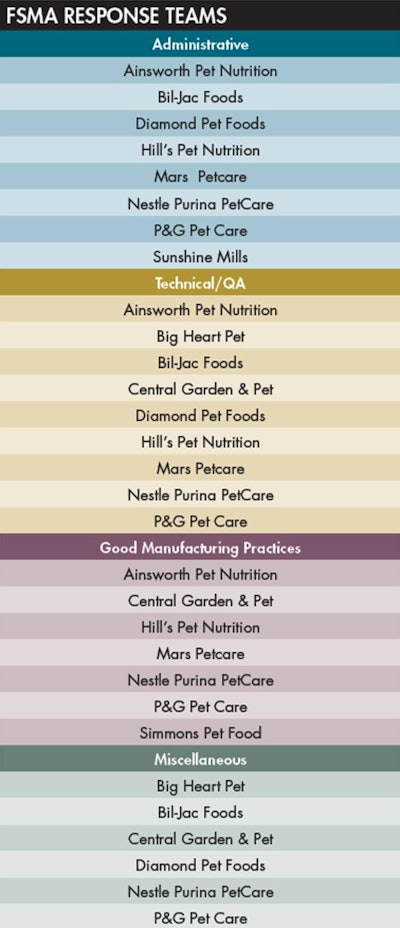
These teams, along with their areas of expertise, included: Administrative (editing and consistency), Technical/QA (hazard analysis, preventive controls and food safety), Good Manufacturing Practices (compare and evaluate versus other established approaches to cGMPs) and Miscellaneous (analyze the appendices, role of testing and non-categorized material). For more information on the composition of the groups, please see “FSMA response teams.”
On October 29, 2013, the rule was published in the Federal Register and the wait was over. Titled, “Current Good Manufacturing Practice and Hazard Analysis and Risk-Based Preventive Controls for Food for Animals,” the rule contained more than 400 pages of text and was indeed virtually identical to the human food rule except that sections on food allergen controls and certain sanitation requirements were replaced with proposed regulations on nutrient imbalance since animals often depend on commercially-made products as their sole source of nutrition.
Following the release of the proposed regulations, the petfood industry’s response plan moved into full force. PFI’s legal counsel reviewed the publication and issued a summary of the most essential aspects of it. The four working groups then began their detailed review of the proposed rule to build industry positions. Each working group met via conference calls and/or webinars at least once a week. PFI hosted several two-day working sessions in Washington D.C. to allow the volunteers to share ideas face-to-face. Another work session was held at the Midyear Meeting of the Association of American Feed Control Officials (AAFCO) in New Orleans, in January 2014.
PFI hosted a webinar in December 2013 with Dan McChesney, director of the Office of Surveillance and Compliance for the Center for Veterinary Medicine, FDA. This event provided information to the industry concerning the thought process FDA used in developing the proposed rule. McChesney then answered dozens of questions from the working group leaders regarding specific provisions and language in the proposed rule.
Each of the teams was then able to consider the complexities found within the rule and, after thorough discussion, develop industry positions that demonstrated group consensus.
Once positions were formed, the drafting process began. After several editing sessions the comments began to look like a final product. In total, more than 80 pages were submitted in response to FDA’s proposal for animal food.
In the final comments, the petfood industry expressed to FDA that while we share the same goals of safe food for animals and we want to work together to achieve that goal, there are some issues in the proposed rule in which we disagree with the agency. Although modelling the animal food rule after the human rule gave us a head-start on the analysis, overall we were disappointed that the animal food rule was based so strongly on human food and does not reflect the distinctions between the two. We urged FDA to provide for sufficient discretion and flexibility to account for these differences.
A good example of this is our concern with FDA’s decision to write new cGMP regulations based on human food practices instead of adopting or incorporating either AAFCO cGMPs or PAS 222:2011. Both of these are programs are already accepted by many within the industry as they were developed specifically for animal food manufacturers with input from FDA.
As one of the co-chairs of PFI’s FSMA response (along with Allen Bingham of Bil-Jac Foods) I can say that I feel very proud to have helped lead this campaign. The dedication of the industry was amazing. Our comments reflect countless hours of careful thought and discussion as to how PFI members will continue striving to produce the safest foods for dogs and cats under FSMA.
FDA releases operational strategy for implementing FSMA: www.petfoodindustry.com/50610.html
AFIA names FSMA work group individuals as Members of the Year: www.petfoodindustry.com/50702.html
Petfood industry’s response to FSMA discussed at Petfood Forum 2014: www.petfoodindustry.com/50377.html