
In January 2013, the Association of American Feed Control Officials (AAFCO) enacted amendments to Model Regulation PF9 regarding calorie content statements on dog and cat food labels. These were published in the 2014 AAFCO Official Publication and became effective as of that time. While AAFCO recommended a grace period for state enforcement, as of June 2015 that period has ended for any newly marketed products (products on the market prior to January 2014 still have until January 2017 to be in compliance). In either case, though, it is prudent to take steps now to ensure labels conform to the new labeling requirements.
Prior to 1995, claims regarding calorie content on pet food labels were simply not permitted under AAFCO Model Regulations. This was because there was no feasible means to guarantee calorie content on the label the same way a label must bear a crude protein or other nutrient guarantee, hence any claim regarding calories could not be effectively verified by regulators. Of course, this lack of a suitable label statement made it difficult for consumers to evaluate diets intended for weight control, because there was no objective basis for them to effectively compare products.
However, the Canine Nutrition Expert/Feline Nutrition Expert Subcommittee (the same folks that developed the AAFCO Dog and Cat Food Nutrient Profiles) devised methods to allow calories to be declared on dog and cat food labels, either by calculation or via digestibility trials. AAFCO subsequently enacted rules to not only allow calorie content statements on all dog and cat food products on a voluntary basis, but also to mandate them on any labels bearing "low calorie," "lite," "reduced calorie" or similarly worded claims.
In 2005, the American College of Veterinary Nutrition (ACVN) took it a step further and submitted a proposal to AAFCO to require calorie content statements on all dog and cat food labels. A major reason for this proposal was to further aide consumers and veterinarians in the fight against pet obesity. Will this change in the rules eliminate fat dogs and cats by itself? Of course not; the issue is far more complex, and admittedly it's likely that many consumers won't take advantage of or even notice that number on the label. However, for those who do want to know and can use this information, it's not unreasonable that it be readily available on the label. While calorie statements on "lite" products are helpful, in practice a wide variety of other foods may be offered to overweight or obesity-prone pets as well, so the need for these statements on all products is warranted.
Further, the need for information on calorie content goes beyond the desire to control calorie intake in pets that are overweight or at risk of becoming overweight. Growing puppies and kittens, working dogs, and animals in gestation and lactation can have a much higher need for calories than the typical sedentary, non-reproducing adult dog or cat. Depending on the life stage and condition, therefore, information as needed to ensure adequate intake of calories can be as important as the need to restrict calorie intake.
Between 2005 and 2013, the proposal was the subject of lively discussion within AAFCO. Of course, with any deliberative process, the specifics within the proposal evolved over the years. In fact, if anything, the final amendments as enacted place more requirements upon the pet food manufacturer than what were originally proposed by ACVN.
Essentially, almost all dog and cat foods must now bear a calorie content statement. This includes not only all complete and balanced foods but also all snacks, treats and non-exempt chew products. It also includes nutritional supplements subject to the AAFCO rules, such as for vitamins, minerals, fatty acids or probiotics. In some cases, such as a mineral supplement, the actual calorie content might be quite low. However, there is no exemption from the requirement for a statement, even if the calorie content of the product is zero.
Rawhides, bones, ears, pizzles and other chews that fully qualify for exemption from AAFCO registration and labeling requirements under Statement for Uniform Interpretation and Policy #27 would not be required to bear a calorie content statement. As with all products falling under this category, it would still have to be labeled as per Food and Drug Administration (FDA) regulations and otherwise not be misbranded, but would not need to bear a calorie content statement, a guaranteed analyses or any other AAFCO-mandated label information.
Also presumably exempt from calorie content statement requirements would be those products labeled as per National Animal Supplement Council (NASC) guidance. Technically, as labeled these products are not "food," hence not subject to AAFCO Model Regulations. Rather, they would be subject to FDA oversight as unapproved drugs of low regulatory priority, as well as oversight by states enforcing animal remedy laws.
Finally, the rules only apply to dog and cat foods. Labels for foods intended for other pets (e.g., birds, reptiles, rodents) do not have to bear a calorie content statement, and in fact may not bear such a statement.
There are three major changes to AAFCO Model Regulation PF9:
- Requires calorie content statements on all non-exempt dog and cat food labels, rather than just mandating them on "lite"-type products.
- Requires expression of values both in terms of kilocalories per kilogram (kcal/kg) and kilocalories per familiar household measure or unit of product (e.g., cans, cups, pieces). Previously, the calories per familiar measure or unit was voluntary, even if a calorie content statement was required. However, while the kcal/kg value helps consumers compare between products, the latter number better assists the consumer in feeding the product appropriately.
- Requires the method of determination ("calculated" vs. "fed") be clearly identified on all statements. In the earlier regulations, the method only had to be identified if the calorie content was determined by the calculation method, otherwise the method did not have to be declared. However, in many cases it was unclear as to whether a statement that did not include "calculated" was actually tested via a digestibility trial or if it was just inadvertently left off the statement.
While the above amendments add to the requirements that manufacturers must follow, other changes to PF9 actually alleviate some of the burden:
- Manufacturers now have more flexibility in the type and amount of data used to calculate calories. The previous rules required proximate analyses be conducted on a minimum of four production batches in order to calculate calorie content. This was particularly difficult for new products, where there might not even have been four batches produced prior to printing the label. The new rules only require that calories be determined by "sound scientific methods," either through laboratory analysis or by calculation from the product formulation.
- Manufacturers can now report the value as determined from the digestibility trial data without restriction. Determination of calories by the calculation method tends to overestimate calorie content of poorly digestible foods and underestimate calorie content of highly digestible foods compared to the digestibility trial method. When the two methods were originally developed, it was decided to put a limit on the reported value derived from the digestibility trial method, because that method is more difficult for the feed control official to verify. In other words, "we trust you, but just so far." Thus, the calories reported on the label from the digestibility trial method could not deviate from that determined from the calculation method by more than 15%. Fortunately, that restriction has gone away, so manufacturers can report a more accurate figure and validate any descriptive term or comparative claim using the true value.
The method for determining calories by the digestibility trial method is well described in protocols in the AAFCO Official Publication. Many testing facilities are familiar with the procedure. Because the animal must be fed the test material as the sole source of nutrition for a minimum of ten days, this method is most appropriate for complete and balanced pet foods rather than treats, chews and supplements.
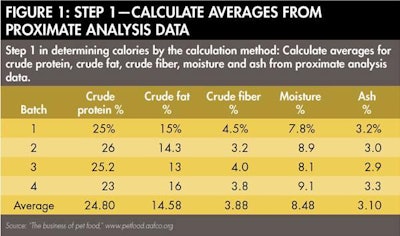
The calculation method is also well described in the AAFCO Official Publication as well as on AAFCO's "The Business of Pet Food" website (www.petfood.aafco.org). In Step 1, average values for percentages of crude protein, crude fat, crude fiber, moisture and ash are determined by proximate analysis or other suitable means (see Figure 1). In the example, proximate analyses of four samples were conducted to obtain the averages. These average values are used in Step 2 to determine the percentage of nitrogen-free extract (NFE), which is simply 100 minus the percentages of the aforementioned components of the proximate analysis (see Figure 2).

By plugging the values for the calorie-containing components of the diet (crude protein, crude fat, and NFE) into the "modified Atwater" formula (with estimated 3.5 kcal/g for crude protein and NFE, and 8.5 kcal/g for crude fat), it generates a value in terms of metabolizable energy (ME) in kcal/100 g. That result is multiplied by ten to give the final answer in terms of kcal/kg, the units as to be reported on the label (see Figure 3).

That's half the battle. Reporting in terms of familiar measure or unit depends on the type of food. Most often, complete and balanced foods should be reported per standard measuring cup of dry food or per can (or other suitable descriptor of the packaging, such as "pouch" or "tray," if applicable) of wet food. Snacks or treats may be reported in discrete units, such as per treat, piece, biscuit or cookie (however it is described elsewhere on the label), while supplements may be "per tablet," "per teaspoon" or as otherwise appropriate. Whatever the unit, first determine the kcal per gram by dividing the number derived in Step 3 by 1000. Then multiply that number by the number of grams per unit (see Figure 4).
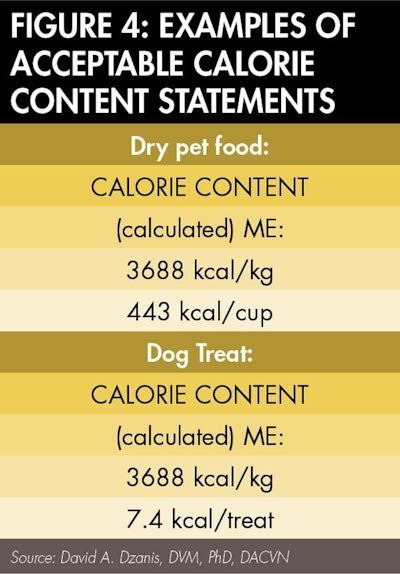
Like the guaranteed analysis, the rules governing calorie content statements allow for some flexibility in format as long as the mandated information is present. Importantly, the statement must be "separate and distinct" from the guarantees. To that end, while not expressly required, it would be advisable to make the header "Calorie Content" the same size, style, color and letter case as the "Guaranteed Analysis" header. Next to or immediately below the header should be the parenthetical "(calculated)" or "(fed)" as appropriate. The statement also should indicate the type of calories as "Metabolizable Energy" or "ME" (this is more for the feed control official's benefit than the consumer's, in order for the former to verify the correct reporting of calories). Finally, of course, the calorie values are reported. Figure 5 shows examples of calorie content statements in an acceptable format.
Although the calculations above often give you very lengthy numbers with many decimal places, on the statement itself it needn't be so precise. Reporting in whole kilocalories is sufficient in most cases. For very small treats or very low calorie items, one decimal place may be prudent, but rarely if ever is any more precision needed. When declaring calories, always rounding up is usually prudent, as it may appear misleading if the calories are underreported.
Because a calorie content statement is based on digestibility trial results or by complex calculation, a feed control official's office cannot verify the accuracy of the statement by laboratory analysis as it may with a crude protein or other guarantee. However, if the numbers are suspicious, the state may request an affidavit attesting to the means by which calories were determined. The affidavit form is in the AAFCO Official Publication. The form further attests that the data used in determining calorie content are on file and available for review by regulators. Of course, if upon laboratory analysis the state finds that you fail to meet your guarantees (e.g., the crude fat does not meet the declared minimum), the average crude fat as reported in the affidavit is likely to be too low as well, which means the accuracy of the calculated calories could also be suspect.
The requirement for calorie content statements on all labels now allow for greater scrutiny of nutritional adequacy statements. Currently, in order for product family members to bear the same statement as the lead product ("Animal feeding tests substantiate..."), the calories in the family member must have been determined by the digestibility trial method. Because the method of calorie determination must now be declared in the statement, it becomes more obvious if the purported family product member was subject to digestibility trial testing or not.
Also, assuming the newly revised AAFCO Dog and Cat Food Nutrient Profiles are passed later this year, there will be a new stipulation that for low-energy foods (presumably anything <4000 kcal/kg DM), meeting the Profiles alone is insufficient proof of nutritional adequacy for gestation/lactation in dog foods and for gestation/lactation or growth in cat foods. So, any product intended for "all life stages" that does not meet this minimum calorie content must be substantiated by means other than meeting the Profiles (e.g., successful passage of a feeding trial, meeting the Product Family criteria).
The new rules for calorie content statements on dog and cat food labels are enforceable now for some products and will become enforceable for all products in the not-too-distant future. However, considering AAFCO enacted these amendments in 2013, hopefully this is not too much of a surprise for most pet food manufacturers. For those who have not made the required label changes yet, now is the time.

















