Vitakraft Sun Seed Inc. of Weston, Ohio, USA recalled one lot of Vitakraft Vita Smart Hedgehog Food because it has the potential to be contaminated with Salmonella, according to the U.S. Food and Drug Administration. To date there have been no reports of illness.
Vitakraft Sun Seed was notified on February 19, 2021, by the Michigan Department of Agriculture that Salmonella was detected in an inspection sample of product from Lot Number 343422. The tests which discovered the bacteria were part of random testing performed by the State of Michigan on consumer products, and not prompted by any consumer concerns.
Products matching the specific lot number below are being recalled:
Vitakraft Vita Smart Hedgehog Food, 25oz
- UPC: 0-51233-34792-9
- Lot: 343422
- Expiration date: 11/06/22
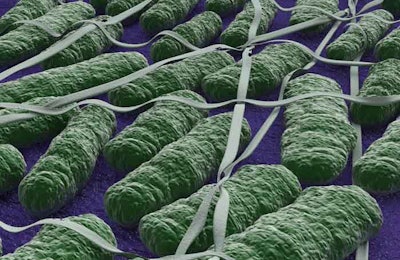
Effects of Salmonella in pet food
Salmonella can affect animals eating the products and there is risk to humans from handling contaminated pet products, especially if they have not thoroughly washed their hands after having contact with the products or any surfaces exposed to these products.
Pets with Salmonella infections may be lethargic and have diarrhea or bloody diarrhea, fever, and vomiting. Some pets will have only decreased appetite, fever and abdominal pain. Infected but otherwise healthy pets can be carriers and infect other animals or humans. If your pet has consumed the recalled product and has these symptoms, please contact your veterinarian.
Healthy people infected with Salmonella should monitor themselves for some or all of the following symptoms: nausea, vomiting, diarrhea or bloody diarrhea, abdominal cramping and fever. Rarely, Salmonella can result in more serious ailments, including arterial infections, endocarditis, arthritis, muscle pain, eye irritation, and urinary tract symptoms. Consumers exhibiting these signs after having contact with this product should contact their healthcare providers.
Retailers and distributors who received the recalled lots have been contacted and asked to pull these lots from their inventory and shelves. Consumers who have purchased a product from the recalled lot should discontinue use of the product and may return the unused portion to the place of purchase for a full refund.


















