Pet food manufacturers are now on the clock to comply with the Current Good Manufacturing Practices and Hazard Analysis and Preventive Controls for Food for Animals rule under the Food Safety Modernization Act (FSMA). After two years developing and revising it, the US Food and Drug Administration (FDA) recently finalized the rule, which goes into effect November 17.
The regulation, commonly referred to as the animal feed preventive control rule, covers all facilities that manufacture, process, hold or pack animal food, said Daniel McChesney, PhD, director of FDA’s Center for Veterinary Medicine Office of Surveillance and Compliance (during the 2015 Feed and Pet Food Joint Conference September 29–October 1 in Columbus, Ohio, USA). That means most pet food manufacturers, though exceptions apply (see #6).
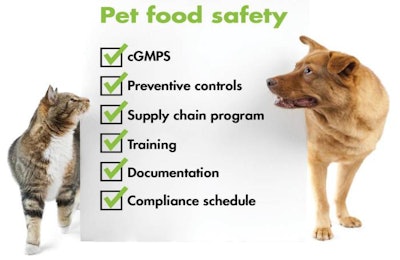
For your pet food facility to comply, here are the key requirements of the rule.
1. cGMPs—current good manufacturing practices. While these have been required for human food for some time, FSMA mandates them for animal feed for the first time. McChesney said that, in response to feedback from industry, plus a review of existing industry standards, FDA made this requirement in the final rule more flexible and less prescriptive because of the wide range of types of facilities and products being made.
The cGMPs requirement, one of two statutory elements of the rule, covers:
- Personnel;
- Plant and grounds;
- Sanitation;
- Water supply and plumbing;
- Equipment and utensils;
- Plant operations;
- Holding and distribution;
- Holding and distribution of human food by-products for use as animal food.
McChesney added that it is up to each facility to determine which of these might have hazards that need control.
2. Preventive controls. Hazards are part of the other statutory element of the rule, hazard analysis and preventive controls. Though also new in terms of a legal mandate, this element should be familiar to most pet food manufacturers because many have been using similar quality and safety programs, such as HACCP.
This element requires animal food facilities to maintain a food safety plan, perform a hazard analysis and institute preventive controls to mitigate those hazards, the rule states. Facilities must also monitor their controls, verify to ensure the controls are effective, take appropriate corrective actions and maintain records documenting these actions. Preventive controls encompass process, sanitation and supply chain controls, plus a recall plan, McChesney added.
3. Supply chain program. This part of the rule essentially matches the Foreign Supplier Verification Program, another FSMA regulation. “Facilities must have a risk-based supply-chain program to ensure control of hazards in raw materials and other ingredients when the control is applied before receipt,” McChesney said. The program should include supplier verification activities (such as on-site audits), sampling and testing, and review of relevant safety records. The activity and frequency should be based on the nature of the hazard, where it is controlled and supplier performance.
4. Training. In the context of this rule, according to McChesney, training means:
- Individuals who manufacture, process, pack or hold animal food must be qualified to perform their assigned duties;
- Qualifications can come from education, training or experience (or a combination);
- The personnel must have training in principles of animal food hygiene and animal food safety;
- Training must be documented.
Scott MacIntire, director of FDA’s Division of Enforcement/Office of Enforcement and Import Operations within the Office of Regulatory Affairs, also spoke at the conference and said the agency has established a Food Safety Preventive Controls Alliance, which is developing training for industry and regulators. Food and feed safety staff from FDA will be required to attend, and industry members who complete the training will receive a certificate, which would fulfill this rule’s requirement.
5. Documentation. “This rule is heavy on documentation; everything must be documented,” McChesney said. That includes the written food safety plan (see #2), any training completed to qualify an individual (#4) and every action the facility takes to comply with each element of the rule.
6. Compliance schedule/exemptions. Pet food manufacturers will have to start following the rule according to a “staggered” compliance schedule. For the cGMPs statutory element, pet food businesses have one year from the effective date (November 17) to comply, with two exceptions: small businesses (fewer than 500 employees) have two years to comply, and very small businesses (less than US$2.5 million in annual sales) have three years. For the preventive controls element, each size of business has an additional year to comply: two, three and four years, respectively.
Enforcing pet food safety: a kinder, gentler FDA?
FDA and industry support to comply with animal feed rule
To help pet food and feed manufacturers comply with the FSMA animal feed preventive control rule, FDA is issuing guidance documents. One on cGMPs is finished, McChesney said, and in the process of being cleared through the federal government’s channels; a document on hazard analysis and preventive controls is under development. See https://goo.gl/mTkcv2.
FDA has also formed a Food Safety Technical Assistance Network to provide central, consistent sources of outreach and real-time technical assistance to industry and regulators before, during and after inspections, MacIntire said. Training for that is under way. The network is in addition to the training that will be offered under the Food Safety Preventive Controls Alliance (#4 in the main article). See https://goo.gl/bDbt2O.
The American Feed Industry Association (https://goo.gl/Y4lGJ8) and AIB International (https://goo.gl/Q1Uqj8) also offer webinars and other training opportunities to the industry.


















