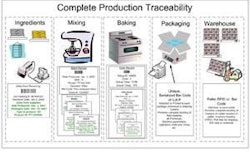
The molecular age is upon us, creating exciting opportunities in the petfood industry. Genome sequence data, high-throughput functional genomics assays and nanotools have several applications to pet health and the petfood industry. Such technology may be used to expand ingredient options (e.g., items enriched in functional nutrients), for petfood testing (e.g., contamination of microbes or toxins; identification of ingredient origin; detection of GMOs) or for molecular-based animal research. Genomic biology may be used to gain a better understanding of dog and cat physiology and how nutrition contributes to health and disease. Because this last point will arguably have the greatest impact on pet health and the petfood industry, it will be the focus here.
Genomic tools have greatly changed the landscape of all research fields pertaining to life sciences, including nutrition. In pets, such tools are being used to study microbial populations and gene expression changes in blood and various tissues.
In the past, the field of microbiology was greatly limited by the methodology and inability to culture the majority of gastrointestinal microbiota. DNA-based techniques, which include quantitative polymerase chain reaction that is very sensitive and used to quantify specific microbial species, gel-based assays such as denaturing gradient gel electrophoresis that allows for a broad view of the population and pyrosequencing that allows for widespread characterization of phylogeny (who is present?) and functional/metabolic capacity (what are they doing?), have many scientific and practical advantages.
Intestinal microbes may be affected by host species, age, health status and location in gastrointestinal tract. Our laboratory and others have begun using DNA-based sequencing techniques to characterize the canine and feline gastrointestinal tracts (Suchodolski et al., 2008; Middelbos et al., 2010; Swanson et al., 2010; Barry, unpublished data). These initial experiments in healthy adult dogs and cats are providing a strong foundation for future initiatives that may be aimed at studying the microbial profile and metabolic pathways of animals with different health status, lifestage, species or dietary regimen.
Genomic biology is also being used to advance our understanding of nutrient-gene interactions within the dog and cat. The field of nutrigenetics is focused on identifying how genetic background affects the response to a nutrient or diet. In contrast, nutrigenomics is focused on measuring how a nutrient or diet affects gene expression. Most mammalian genomes contain 20,000-25,000 genes, all interacting in complex ways. Identifying the genetic basis for disease may be difficult, even for monogenic diseases affected by a single mutation.
Monogenic diseases have been identified in dogs and cats, but many are not directly related to diet. Moreover, while mutations of several monogenic diseases have been identified, the majority of diseases noted in pets are complex in nature. Diseases such as obesity or hip dysplasia, for example, are impacted by hundreds of genes and numerous environmental factors, including diet. While this field has enormous potential, we are many years away from devising a personalized food for any given dog or cat based solely on a blood (DNA) sample.
The field of nutrigenomics continues to enhance our understanding of canine and feline metabolism and has broad application to pet nutrition and health. Recently, researchers have applied canine- and feline-specific functional genomic assays to study adipose and skeletal muscle tissue biology, various disease states, the normal effects of aging and diet-induced changes in gene expression.
To lay the foundation for future studies in diseased or aged dogs, our lab initially focused on identifying gene expression differences in cerebral cortex (Swanson et al., 2009b), skeletal muscle (Middelbos et al., 2009), adipose (Swanson et al., 2009a), colon (Kil et al., 2010) and liver (Kil, unpublished data) tissues of healthy aged vs. young adult dogs. Biological systems highlighted in these datasets have provided targets of nutritional intervention in future projects focused on improving tissue function and/or longevity.
Our lab and others have also used genomic biology to identify molecular changes in adipose and skeletal muscle tissues during weight gain/loss, after spay/neuter or in obese cats and dogs (Leray et al., 2008; Belsito et al. 2009; Vester et al., 2009a; Vester et al., 2009b). These studies have suggested the potential role of specific nutrients or phytochemicals, such as estrogenic-like compounds or green tea polyphenols, that may aid in healthy weight maintenance and deserve more attention in future research. Similarly, Brass et al. (2009) measured temporal gene expression patterns of skeletal muscle in Alaskan sled dogs to identify novel regulatory mechanisms and responses to exercise stimuli, which may lead to dietary strategies with the ability to prolong bouts of exercise or improve muscle recovery.
Finally, other researchers have identified key genes or pathways differentially regulated in diseases such as atopic dermatitis (Merryman-Simpson et al., 2008) and gastrointestinal diseases (Greger et al., 2006). In addition to highlighting genes or pathways associated with disease that may aid in developing preventive or treatment strategies, such studies have suggested that genomic tests may also be an effective diagnostic tool to distinguish closely-related diseases from one another (Greger et al., 2006).
Genomic biology is rapidly changing the research environment, providing for new and exciting avenues of research geared toward improving the health or longevity of pets. Even though this field is in its infancy, initial publications have shown great promise, highlighting dietary strategies for further testing or inclusion in petfoods.