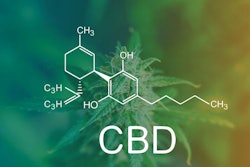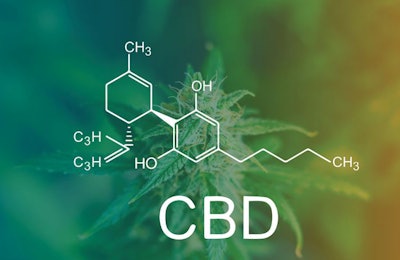
CBD-derived pet foods and treats currently occupy an interesting regulatory space. The U.S. Food and Drug Administration (FDA) has said that any animal feed or pet food containing cannabidiol (CBD) is illegal under federal law, yet the market is flooded with CBD-infused pet products. FDA is under pressure from stakeholders to find a regulatory path forward for CBD-containing foods. Until then, however, it is a bit of the regulatory wild west.
Even though there may be uncertainty on the regulatory status of CBD products, responsible companies that are selling CBD-containing products should know where FDA has been focusing its enforcement. Understanding the bounds of prior FDA actions may help inform the marketing strategy of companies entering the burgeoning CBD pet food market.
FDA enforcement against companies selling CBD-containing pet foods: a survey of published warning letters
Since 2015, FDA has issued 23 warning letters to companies selling CBD-derived human and animal products. “Warning letters” are administrative actions that are akin to cease and desist letters. Warning letters typically are addressed to a company’s CEO, and receive a fair amount of press attention because FDA publishes them on its website each week.
Of the 23 total letters, four were issued to companies selling pet foods. Two of those letters were issued in 2019, including one in July, and two were issued in 2015. Warning letters for human products have been less sporadic — the majority of the 23 letters cited hemp or CBD-derived human ingestible products such as oils, tinctures, gummies, lollipops and capsules. One-third of the letters mentioned human topical products, such as salves and lotions, and about one-quarter of the letters discussed vape products (e.g., e-liquid or vape pens).
Guideposts from the warning letters: marketing CBD pet foods
FDA has stated that it does not plan on exercising a policy of enforcement discretion with respect to any CBD products, yet FDA has not aggressively pursued marketers of CBD products. Based on the warning letters issued to date, the following are guideposts that legal and marketing teams should consider as they advise on strategies for promoting their products:
- Avoid “over-the-line” therapeutic claims.
A clear takeaway from the letters is that FDA’s focus is on therapeutic claims for diseases and serious conditions. Claims that CBD can treat diseases such as arthritis, psoriasis, asthma and cancer are a major red flag to FDA. - Avoid “pain,” “anxiety” and other “drug-like” claims.
Like therapeutic claims, claims about treating or alleviating symptoms such as “pain,” “inflammation” or “anxiety” are likely to be considered impermissible “drug-like” claims for pet products. - Claims on social media and testimonials are fair game.
All of the warning letters include claims from the company’s website and nearly all of the letters cite statements on social media sites (e.g., Facebook or Twitter). For instance, in the context of a canine CBD capsule, FDA objected to the following testimonial from a company representative: “Good news about Cyndi … She’s a 14-year-old lab who has spinal arthritis and a type of cancer called adenocarcinoma. Miss Cyndi is responding well to her [CBD product].” Also, in the context of CBD-containing products for humans, FDA objected to customer testimonials. For example, FDA cited customer testimonials such as: “I was pleasantly surprised to find that CBD helped my arthritis,” and “I have been severely depressed … [so] I started taking this [CBD oil] product … I have gone from basically clinically depressed to feeling okay about life.” Website and social media sites are easy for regulators to access remotely, and companies should be monitoring posts on their social media sites to ensure impermissible drug-like claims — whether explicit or implied — are not being posted. - There is no such thing as a “dietary supplement” for a pet.
FDA separately objected to products promoted as dietary supplements. Dietary supplements are a category of food products that allow certain health claims, but supplements are defined by statute to be only intended for humans. The only category of pet products that allows similar claims are animal drugs. (Dietary supplements containing CBD also are not currently permitted by FDA.)
Joining the CBD product trend may be lucrative, but companies should use caution and consult with qualified counsel to avoid potential pitfalls and enforcement actions.
Read the companion article, "CBD 'boom' brings pet product regulatory issues to light,” published in the December 2019 issue of Petfood Industry magazine.