Last year, the US Food and Drug Administration (FDA) finalized the Current Good Manufacturing Practices and Hazard Analysis and Preventive Controls for Food for Animals rule under the Food Safety Modernization Act (FSMA). The rule went into effect November 17, 2015. The regulation, commonly referred to as the animal feed preventive control rule, covers all facilities that manufacture, process, hold or pack animal food. One key requirement of the rule is training.
FDA has developed the standardized Animal Food Preventive Control Qualified Individuals (AFPCQI) curriculum to train mill personnel and regulators as one way to become qualified to oversee the required food safety plans and other required activities related to the FSMA.
Classes teaching the AFPCQI curriculum are now available from Feed PC Training Inc. Feed PC Training Inc. posted dates for when they will teach the AFPCQI curriculum in Colorado, Tennessee, Oregon and Hawaii. The AFPCQI needs to be qualified either by experience or training to develop and apply risk-based preventive controls. They oversee preparation of the food safety plan, validation of any preventive controls, review of records, reanalysis of the food safety plan, along with several other tasks required under FSMA.
The course runs two-and-a-half days. Upon completion participants will receive a certificate from the Food Safety Preventative Controls Alliance. Participants must be present the entire 20 hours to receive a certificate.
The American Feed Industry Association will host a Food Safety Preventive Control Alliance (FSPCA) Preventive Controls for Animal Food training session in Auburn, Alabama from August 16 to 18. The training material was developed by FSPCA and is the standardized curriculum that the US Food and Drug Administration recognizes as adequate for preventive control qualified individual (PCQI) training. Successfully completing this course is one way to meet the requirements for a PCQI.
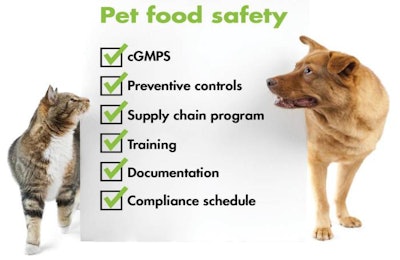
The other five key requirements of the animal feed preventive control rule are:
- Current good manufacturing practices (cGMPs) -- While these have been required for human food for some time, FSMA mandates them for animal feed for the first time. The cGMPs cover personnel, plants and grounds, sanitation, water supply and plumbing and other aspects of production and distribution.
- Preventive controls -- This element requires animal food facilities to maintain a food safety plan, perform a hazard analysis and institute preventive controls to mitigate those hazards, the rule states. Facilities must also monitor their controls, verify to ensure the controls are effective, take appropriate corrective actions and maintain records documenting these actions.
- Supply chain program -- The program should include supplier verification activities (such as on-site audits), sampling and testing, and review of relevant safety records. The activity and frequency should be based on the nature of the hazard, where it is controlled and supplier performance.
- Documentation - Everything must be documented, including the written food safety plan, any training completed to qualify an individual and every action the facility takes to comply with each element of the rule.
- Compliance schedule/exemptions - Pet food manufacturers will have to start following the rule according to a staggered compliance schedule.

















