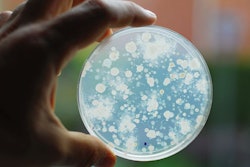
As of October 30, 2019, officials believe the Salmonella outbreak connected to pig ear pet treats seems to be over. The U.S. Food and Drug Administration (FDA) and Centers for Disease Control (CDC) started their investigation of the outbreak in July. CDC now reports that the rate of new human infections has returned to pre-outbreak, baseline levels.
Pig ear pet treat sale and use
“Based on the slowed rate of human illness reports, the FDA and CDC are no longer recommending that people avoid purchasing or feeding pig ear pet treats entirely,” FDA officials wrote in a press release.
At the end of July, FDA and CDC recommended no pig ear pet treat sale or use in the United States. With the end of the outbreak, the FDA altered its guidance to pet product retailers and pet owners. The agency now recommends that retailers who wish to re-introduce pig ear pet treats should take appropriate steps to ensure that their suppliers are controlling for pathogens such as Salmonella, and that products are not cross-contaminated after processing. Likewise, the agency advised pet owners to use good hygiene when feeding pig ear pet treats.
History of Salmonella outbreak and pig ear pet treats
Reports of illness from these Salmonella infections started on June 10, 2015 and ran until September 13, 2019. Over the course of the outbreak, official reports tied 154 cases of human infection with exposure to pig ear pet treats in 34 states. Patients ranged in age from less than one year to 90 years. Of 133 cases with info available, 35 people needed hospitalization. Children younger than 5 years were infected in 27 cases.
Public health officials conducted genome sequencing of the Salmonella involved in the outbreak. The researchers revealed that many of the strains were resistant to multiple antibiotics, including ampicillin, streptomycin, tetracycline and ciprofloxacin. Salmonella strains identified were Cerro, Derby, London, Infantis, Newport, Rissen and I 4,[5],12:i:-.
More information from FDA press release:
Three firms recalled product associated with the outbreak: Pet Supplies Plus, Lennox, and Dog Goods USA. A fourth firm, Hollywood Pet, also recalled Salmonella positive pet ear treats that it had sourced from Dog Goods USA, but testing was not sufficient to determine if these treats were connected to illnesses. All of these recalled products originated from suppliers in Argentina, Brazil and Colombia. The importers were placed on Import Alert 72-03 (“Detention Without Physical Examination and Intensified Coverage of Pig Ears And Other Pet Treats Due To The Presence of Salmonella”). These importers were Suarko, SRL (Argentina) and Anabe Industria e Comercio de Proteinas (Brazil), and Custom Pet S.A.S. (Colombia).
In the course of the investigation, testing identified two additional products that tested positive for strains of Salmonella. These products were not associated with the outbreak and no illnesses have been reported. One of these firms sourced product from Colombia. The second firm sourced product within the United States, and the recalled product had been irradiated. Both distributors (Brutus & Barnaby, TDBBS) recalled these products.