Work on updating and improving two key sections of the pet food label required in the US is moving at a strong, steady pace through the Association of American Feed Officials Organization’s (AAFCO) approval process, said Jim Barritt, government and regulatory affairs manager for Mars Petcare. He provided an update on the project during the eighth annual Feed and Pet Food Joint Conference, held October 23-25 in Louisville, Kentucky, USA.
Efforts to improve the US pet food label have been under way for more than two years, originally through a task force convened by the Pet Food Institute (PFI) and headed by Barritt. He first reported on the task force’s work during the 2015 joint conference, which is organized by PFI and the National Grain and Feed Association.
At that time, Barritt said the task force’s work arose from 2013 consumer research that Mars conducted to try and influence potential new pet food labeling rules to come from the US Food and Drug Administration (FDA) as part of the FDA Amendment Act, passed in 2007 in the wake of the melamine-related pet food recalls. The research pinpointed several sections of the pet food label that consumers found the most confusing, or just ignored altogether.
Good progress on nutritional statement, Pet Nutrition Facts box
As Barritt’s PFI task force was doing its work, FDA approached AAFCO asking for input and help on pet food label improvements, meaning the task force was able to offer ideas and participate in AAFCO’s efforts almost immediately. That eventually led to the formation of an AAFCO pet food label modernization working group, with a sub-group for each of four areas identified by the Mars research and chosen by AAFCO for improvement: the nutritional adequacy statement, guaranteed analysis, ingredients list, and safety and handling statement.
While work on the latter two areas seems to be moving at the glacial pace typical of AAFCO, Barritt reported that proposals for new treatments of the nutritional adequacy statement and guaranteed analysis have been presented twice, with little pushback to date, to AAFCO’s Pet Food Committee. He expressed pleasant surprise at how quickly the work is progressing.
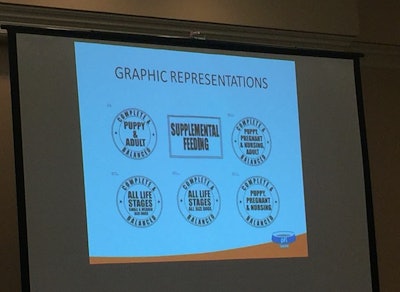
Graphic representation of nutritional adequacy
As we know, the current method for declaring that a pet food product is complete and balanced is with a required statement – usually buried on the back of the package – reading something like, “Formulated to meet the nutritional levels established by AAFCO feeding trials for all lifestages.” Which means nothing to consumers.
Under the proposal moving through AAFCO, that statement would still appear on the back of the package, but on the front, probably in the lower right quadrant of the package, would also appear a “graphic representation” (AAFCO doesn’t like the term or concept of a seal, Barritt said) of the statement in much plainer language. Around the edges of a circle, for example, the words “complete and balanced” would appear, while inside the circle would be text such as “all lifestages” or “puppy, pregnant and nursing adult” or “all lifestages, small and medium-size dogs.”
The goal with the proposed language is to get away from terms such as “growth” and “maintenance” that may not be clear to consumers. A product not intended to be complete and balanced, such as pet treats, would bear a symbol, probably in a different shape (perhaps a rectangle), saying “supplemental feeding.”
The symbol or graphic representation on the front of the package would be required, along with the statement on the back. Barritt showed some concepts currently under consideration. He doesn’t know if the final versions, if approved, would look exactly like them, but he does believe they would have white backgrounds with black text; and there would be requirements for the size of the symbol relative to the size of the package.
From guaranteed analysis to a familiar facts box
One of the worst pain points for consumers identified by the 2013 Mars research was the guaranteed analysis on pet food labels. Certain terms, such as “crude” protein, have a negative connotation (“Do consumers even need to know this?” Barritt asked. “It’s really about the lab method”), and the minimum and maximum levels are also very confusing because they don’t add up to 100 percent. The list of “non-required ingredients” also perplexes consumers; in the research, some wondered why they are even in pet food if they’re not required?
In response, the PFI task force “thought inside the box,” Barritt said, proposing a Nutrition Facts Box similar to the one used on human foods for some time. While consumers may still not fully understand all the information in the box on human foods, at least it’s familiar, and many people are starting to educate themselves on the various elements.

In addition, the task force suggested dropping the “crude” term, as well as minimums and maximums. AAFCO seems to be OK with all so far except the latter, though under the proposal, they would be expressed as guaranteed amounts for each nutrient, rather than “analysis.” Also, to avoid confusion with human food labels, AAFCO asked that the box be labeled Pet Nutrition Facts.
Other elements of the box include using text that indicates intended use – e.g., for all lifestages – to bolster the symbol on the front and the AAFCO statement. The box would list a standard reference unit (one cup for dry pet food, one can or tray for wet or other formats), along with weight or calories per cup or unit to address the wide variance in bulk densities among pet foods while still giving consumers a reference to compare foods by calorie level. It would also list calories by percentages among the major nutrient categories (protein, fat, carbohydrate).
Timeline for pet food label changes
Despite Barritt’s optimism over the relatively fast progress for these two sections of the label, there is still likely a long road ahead before any changes are approved. First, changes to the other two sections need to be proposed by their respective sub-working groups. Barritt is hopeful that could happen in January at AAFCO’s mid-year meeting and said there is an impetus to meet that timeline because AAFCO sees an opening to make changes while all the states are reviewing their feed and pet food regulations respective to regulations under the Food Safety Modernization Act.
Yet all final changes would need to be voted on and accepted by the Pet Food Committee, then AAFCO’s Model Bill Committee, then they might be kicked back to the Pet Food Committee – and they would have to go through the AAFCO board of directors in between all those steps. Once finally approved by all those entities, the changes would need to be voted on by AAFCO’s general membership.
Plus, this would be an extensive change to labels for pet food companies, so AAFCO would need to allow for a reasonable compliance period, possibly up to three years. The cost to companies to change all their product labels makes it even more important that all the changes, for all affected sections, are approved and communicated together.
Still, the work accomplished so far, and its apparent acceptance, represents welcome progress for a change that could significantly help pet owners in choosing and purchasing foods for their pets.