
Product claims are nothing new to the human or pet food industries. In fact, it is not unheard of to see health claims in both categories. Some people may have you believe that health claims are unique only to the pet food category; however, the Food and Drug Administration (FDA) has allowed health claims on human products since 1990 (FDA, 2017a). Additionally, both the human and pet food industries share much of the same claim types and hierarchies of required proof.
Pet food claims 101
Originally, I was going to write a single blog post about pet food product label claims; however, following my recent attendance at the Association of American Feed Control Officials (AAFCO) Pet Food Labeling Workshop in Bellevue, Washington, USA, I thought a single blog post would serve the industry a huge injustice. Although one would think a room full of regulatory people and issues would be the last place you would want to hang out, I found this to be one of the most informative meetings I have attended in a long time. Topics ranged from the basics, such as ingredient statements and guaranteed analysis, to the more complicated and often debatable product claims.
If you do not have the ability to attend one of the sessions, I suggest you purchase the AAFCO Pet Food Labeling Guide. This book is easy to follow and is a good back-up in “goof-proofing” your claims.
How do I decide what type of claim to make?
The short answer, it depends. Do you want to make hard-hitting and differentiating science-based claims, or do you want to make “belly button” claims (i.e., everybody has one). I think it is fair to say that if you are making meat-first, natural, grain-free or farm-to-table claims and think you are differentiating, then you need to take a hard look at your marketing team.
If you are wanting to make differentiating claims that stand out from the pack and make your product or brand unique, then you need to understand the different types, levels of claims and their requirements. By understanding these levels and requirements, you can prevent or delay your competition from making similar claims while staying in regulatory compliance. These claims can be anything from supply chain, novel ingredients, nutritional or scientific categories, to name a few.
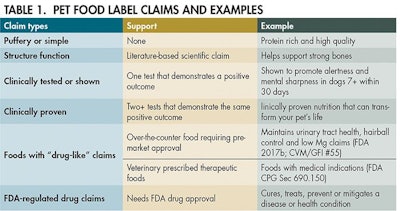
Pet food product claims
Since there are many claim types, I decided to break up my discussion of product claims into a multipart series. Also, it is important to recognize that some claims are not always reflective of FDA or AAFCO’s regulations or guidance and may result from outside organizations and agencies. For example, claims like “Made in the USA” must meet the guidance set forth by the Federal Trade Commission. Organic and humanely raised must meet the requirements and be certified by the National Organic Program (USDA-AMS) or certain non-government organizations. Since scientific claims can fall into all the categories, I will focus on these claim types in this blog post.
If you want to make differentiating claims that stand out from the pack and make your product or brand unique, then you need to understand the different types, levels of claims and their requirements.
Puffery to drugs
The simplest and least burdensome scientific claims are generally classified as puffery claims. Puffery claims are typically general (vs. literal), objective and are hard to prove or disprove. I like to use the farm-to-table claim as an example. Some companies can track their food supply down to farmer Joe in Kansas. While other companies may not be able to get down to that level of detail, they can make similar claims because chickens and rice come from farms. It can imply higher quality or healthier products, depending on the consumer.
Just remember, if you make this claim, be prepared to explain your supply chain to your consumer. I will dive deeper into this claim in future blogs since it also has other meanings and implications and seems to be the new popular claim.
Drug claims are on the other end of the spectrum. If your food makes any claims to treat, prevent, cure or mitigate a disease (or an implied disease like inflammation), then you will likely fall into the drug category.
Proof behind the scientific claims
Therapeutic foods require veterinary oversight, must not present a known safety risk, not include representations of disease or treatment on the product label and not be marketed as an alternative to approved new animal drugs (in addition to regular pet food labeling requirements). Although over-the-counter foods may utilize similar claims, therapeutic foods generally use published (structure function claim) and clinical studies to support their claims.
What does clinical really mean?
Most companies like to use the word “clinical” when they describe the study or studies, but what does it mean? To the consumer it can mean white lab coats, a sterile setting like a veterinary clinic and hundreds to thousands of dogs or cats on the study. To the researcher and person developing the claims, it simply means a group of dogs or cats tested the product or nutrition in an experimental setting (i.e., a kennel).
Don’t believe me?
Just check out the minimum feeding protocol for proving an adult maintenance claim for a dog or cat food (AAFCO, 2017). If I run that study, I can make the claim clinically shown to maintain weight. If I run two or more of those studies using the same food, guess what? I am clinically proven now. Depending on what you measure as outcomes of the study, the claim could be tied to weight loss, blood parameters, mobility, etc.
Additionally, if the claim says clinically tested or proven nutrition, then the consumer is not feeding the actual food tested – simply the same nutrition. For example, if it is tied to a certain protein level, then the food in the marketplace may only have the same protein level, not the other nutrients or ingredients that were actually tested in the study.
Also, I would be remiss if I didn’t mention that the same eight-dog or cat study is what gives companies the claim, Animal feeding tests using AAFCO procedures substantiate that Product XYZ provides complete and balanced nutrition for canine (or feline) adult maintenance.
Next topic: Navigating through product claims: part 2
Next time we will discuss this topic: “Navigating through product claims: part 2.” If there are topics you would like to have discussed, feel free to comment below or reach out via LinkedIn: www.linkedin.com/in/ryanyamka.
References
AAFCO. 2017. Association of American Feed Control Officials: Official Publication (pages 174 to 178).
AAFCO Pet Food Labeling Guide Date Accessed: October 7, 2017. https://www.aafco.org/resources/guides-and-manuals/good-test-portions-and-goodsamples-resources/
CVM/GFI#55 Date Accessed: October 7, 2017. https://www.fda.gov/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/ucm053415.htm
FDA, 2017a. Nutrition Labeling and Education Act (NLEA) Requirements. Date Accessed: October 7, 2017. https://www.fda.gov/ICECI/Inspections/InspectionGuides/ucm114092.htm#ATTACHMENT_4
FDA, 2017b. Pet Food. Data Accessed: October 7, 2017: https://www.fda.gov/animalveterinary/products/animalfoodfeeds/petfood/default.htm
FDA, 2016. Compliance Policy Guide. Sec. 690.150 Labeling and Marketing of Dog and Cat Food Diets Intended to Diagnose, Cure, Mitigate, Treat, or Prevent Diseases. Date Accessed: October 7, 2017. https://www.fda.gov/downloads/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/UCM318761.pdf



















